
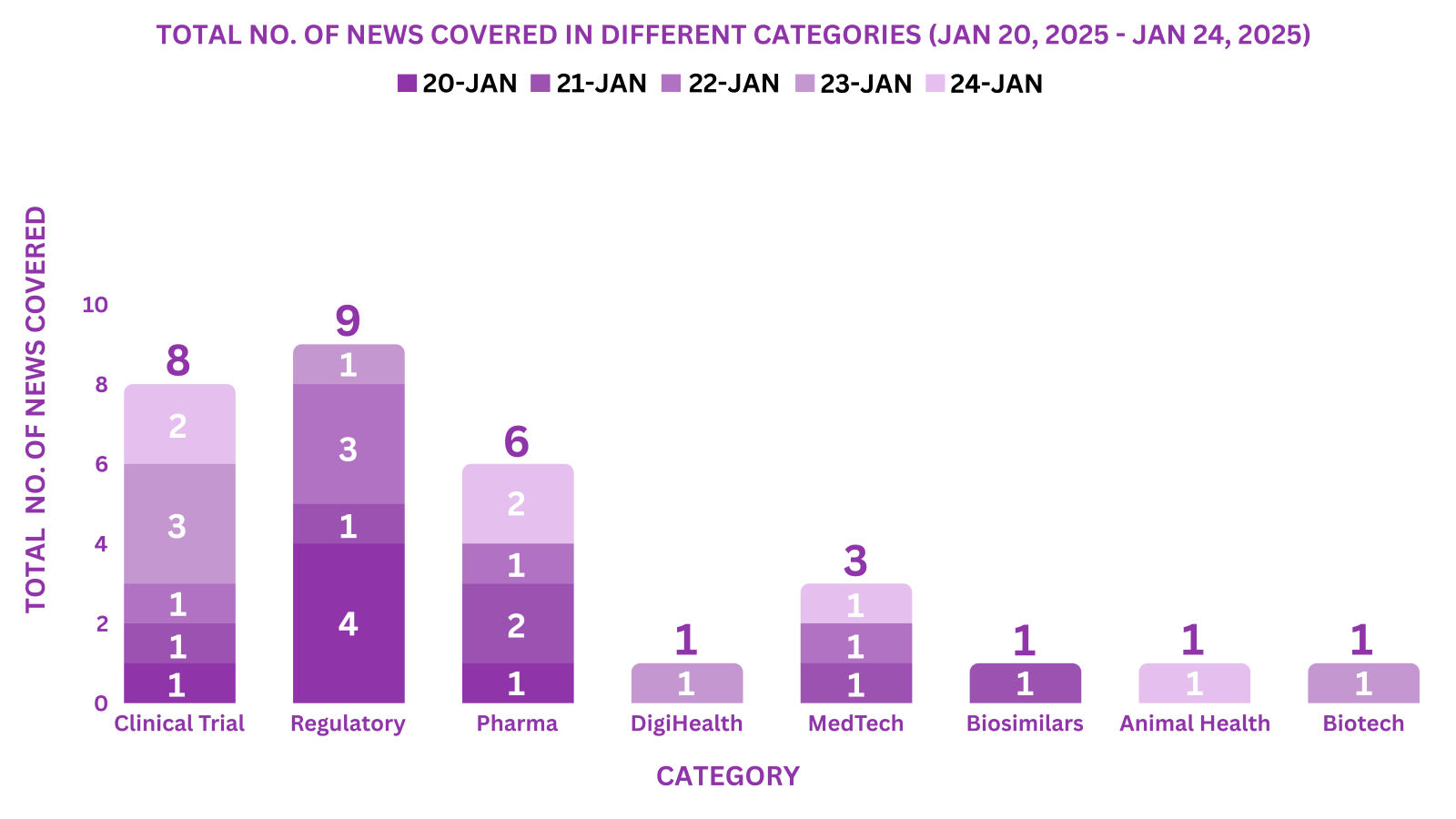
PharmaShots Weekly Snapshots (January 20, 2025 – January 24, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, DigiHealth, MedTech, Biosimilars, Animal Health & Biotech. Check out our full report below:

Novo Nordisk Reports Headline Results from P-IIIb (STEP UP) Study of Semaglutide 7.2mg in Obese Adults without Diabetes
Read More: Novo Nordisk
Yoltech Initiates a Dose-Escalation Study for YOLT-204 to Treat β -Thalassemia
Read More: Yoltech
Antennova to Highlight P-I/II (CLINCH) Trial Data of ATN-022 to Treat Advanced/Metastatic Gastric Cancer at ASCO GI 2025
Read More: Antennova
Coherus to Highlight Final P-II Study Data of Casdozokitug Regimen to Treat Metastatic Hepatocellular Carcinoma at ASCO-GI 2025
Read More: Coherus
Tris Pharma Reports the P-III (ALLEVIATE-1) Study Results of Cebranopadol to Treat Moderate-to-Severe Acute Pain
Read More: Tris Pharma
Agenus Highlights Data from Five Studies of Botensilimab/Balstilimab (BOT/BAL) Regimen as Multiple lines of Therapy in Colorectal Cancer at ASCO GI 2025
Read More: Agenus
Rakuten Medical Commences Global P-III (ECLIPSE) Study of ASP-1929 Plus Keytruda as a 1L Treatment of Recurrent Head and Neck Cancer
Read More: Rakuten Medical
Lisata Therapeutics and WARPNINE Reports Initial Results from P-Ib/IIa (iLSTA) trial of Certepetide Regimen in Pancreatic Ductal Adenocarcinoma (PDAC)
Read More: Lisata Therapeutics and WARPNINE

Amgen Receives the US FDA Approval for Lumakras + Vectibix to Treat Chemorefractory KRAS G12C-Mutated Metastatic Colorectal Cancer (mCRC)
Read More: Amgen
Daiichi Sankyo and AstraZeneca Receives the US FDA Approval for Datroway (Datopotamab Deruxtecan-dlnk) to Treat HR+/HER2- Metastatic Breast Cancer
Read More: Daiichi Sankyo and AstraZeneca
AstraZeneca’s Calquence (Acalabrutinib) Plus Chemoimmunotherapy Secures the US FDA’s Approval for Mantle Cell Lymphoma (MCL)
Read More: AstraZeneca
GSK’s Jemperli Plus Chemotherapy Receives the EC’s Approval as a 1L Treatment of Primary Advanced or Recurrent Endometrial Cancer
Read More: GSK
Takeda Receives Health Canada’s Approval for Fruzaqla (fruquintinib) to Treat Metastatic Colorectal Cancer (mCRC)
Read More: Takeda
The US FDA Approves sNDA for J&J’s Spravato (esketamine) to Treat Major Depressive Disorder (MDD)
Read More: J&J
The EC Approves Sanofi’s Sarclisa + standard-of-care VRd to Treat Newly Diagnosed Multiple Myeloma (NDMM)
Read More: Sanofi
Solid Biosciences’ SGT-212 Secures the US FDA’s Fast Track Designation for Treating Friedreich’s Ataxia
Read More: Solid Biosciences
Zai Lab’s ZL-1310 Secures the US FDA’s Orphan Drug Designation to Treat Small Cell Lung Cancer (SCLC)
Read More: Zai Lab

Fermion Technology and Simcere Pharma Join Hands to Develop FZ002-037 for Pain Management
Read More: Fermion Technology and Simcere Pharma
InnoCare and KeyMed Together Partner with Prolium Bioscience to Develop ICP-B02
Read More: InnoCare and KeyMed
Junshi Biosciences & LEO Pharma Collaborate to Commercialize Toripalimab in Europe
Read More: Junshi Biosciences and LEO Pharma
ArriVent Biopharma Collaborates with Lepu BioPharma to Develop and Commercialize MRG007 for the Treatment of Gastrointestinal Cancers
Read More: ArriVent Biopharma and Lepu BioPharma
Abbvie Partners with Neomorph to Develop Novel Molecular Glue Degraders for Oncology and Immunology
Read More: Abbvie and Neomorph
IMMvention Therapeutix Partners with Novo Nordisk to Develop Oral BACH1 inhibitors for Chronic Conditions
Read More: IMMvention Therapeutix and Novo Nordisk

Holon Health Reports Development of Holon Vibe App for the People with Substance Use Disorders (SUD)
Read More: Holon Health

The US FDA Approves Innovate’s MediBeacon Transdermal GFR System to Evaluate Renal Function
Read More: Innovate
Roche Receives 510(k) Clearance & CLIA Waiver for Cobas Liat Multiplex Assay Panels to Diagnose Sexually Transmitted Infections
Read More: Roche
Medidata (Dassault Systèmes brand) and Tigermed Expand their Strategic Collaboration to Expedite Clinical Trials Globally
Read More: Medidata and Tigermed

Formycon Reports the EC’s Approval of FYB203 (Biosimilar, Eylea) Under the Brand Names Ahzantive and Baiama
Read More: Formycon

Virbac Launches Zenifel Pheromone Product to Address Stress-Related Behaviors in Cats
Read More: Virbac

Tiziana Life Sciences Identifies New Immune Biomarkers in Multiple Sclerosis Patients Treated with Foralumab
Read More: Tiziana Life Sciences
Related Post: PharmaShots Weekly Snapshots (January 13, 2025 – January 17, 2025)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














